Most researchers are pretty circumspect about the potential for ketogenic diets or exogenous ketone bodies to cure cancer, largely because the disease is so heterogenous. On the other hand, as an adjunct to other forms of treatment, there is great promise and the literature already has many examples. We are studying the effect of ketone bodies on the killing of cancer cells in culture by external compounds, rapamycin (which targets the global effects of mTOR), methotrexate (inhibits DNA synthesis by targeting folic acid for thymidine synthesis). Of particular interest is a novel compound, PNC-27, known to kill cancer cells but with no effect on normal cells. The figure below shows a typical experiment. PNC-27 kills MCF-7 (breast cancer-derived) cells. When done in the presence of lithium acetoacetate, the effect is enhanced due to the effect of both the ketone body and the lithium ion. The idea is that if PNC-27 can really be developed as a clinical treatment, lower doses could be used if, in addition, the patient were on a ketogenic diet. The figure shows MCF-7 cells treated with increasing concentrations of PNC-27. Cell growth was determined with crystal violet, a dye that stains only living cells. Experiments were done in the absence (green) or presence of lithium acetoacetate (blue). It is clear that ketone bodies (as lithium salt) can reduce the level of PNC-27 needed to kill the cells.
PNC-27 was designed by my coworker, Matthew Pincus. He had a sophisticated plan, hoping to take advantage of the complex features of the cell’s metabolism — the action of tumor suppressor genes, the cell cycle and regulation of protein destruction. Here, I am going to use the idea behind PNC-27 to bring out these various features of cell biology. Spoiler alert: at the end, I will tell you that this brilliant strategy was not exactly played out. I will show you how PNC-27 really works and I will describe how we lucked out and got a great potential drug. So, if you follow this post, it will turn out that you will have become educated in cell biology just for the hell of it.
The strategy.
Good advice: Mike Eades said he learned it from one of his teachers but early on I figured out for myself that the key to success in research lies in having collaborators and students who are smarter than you are. That’s why I follow Mike’s writing (notwithstanding a few disagreements ) and why I have been working with Matthew Pincus. He was a graduate student when I started at Downstate Medical Center. On graduation, he did a post-doc with Harold Scheraga and they published uncountable important papers on protein structure. He came back to Downstate and received an MD and is now a pathology professor. Matthew set out to find a drug that would cure cancer. He is a very creative scientist, very knowledgeable about chemistry and medicine. But sometimes it takes more than that. Napoleon said that good luck is also a character trait and that turned out to be part of his personality.
Oncogenes and tumor suppressors.
In this game, there are two major kinds of genes that affect the cancerous state, oncogenes and tumor suppressors. Oncogenes represent mutated genes that predispose to cancer. The living cell runs on timing cycles, making new material and duplicating itself at appropriate times. Some oncogenes disrupt this timing and the cell keeps proliferating when it should stop and switch to the next part of the cycle. Tumor suppressor genes, on the other hand, do what it sounds like. They generally keep the cell on track. If they are mutated they lose this ability.
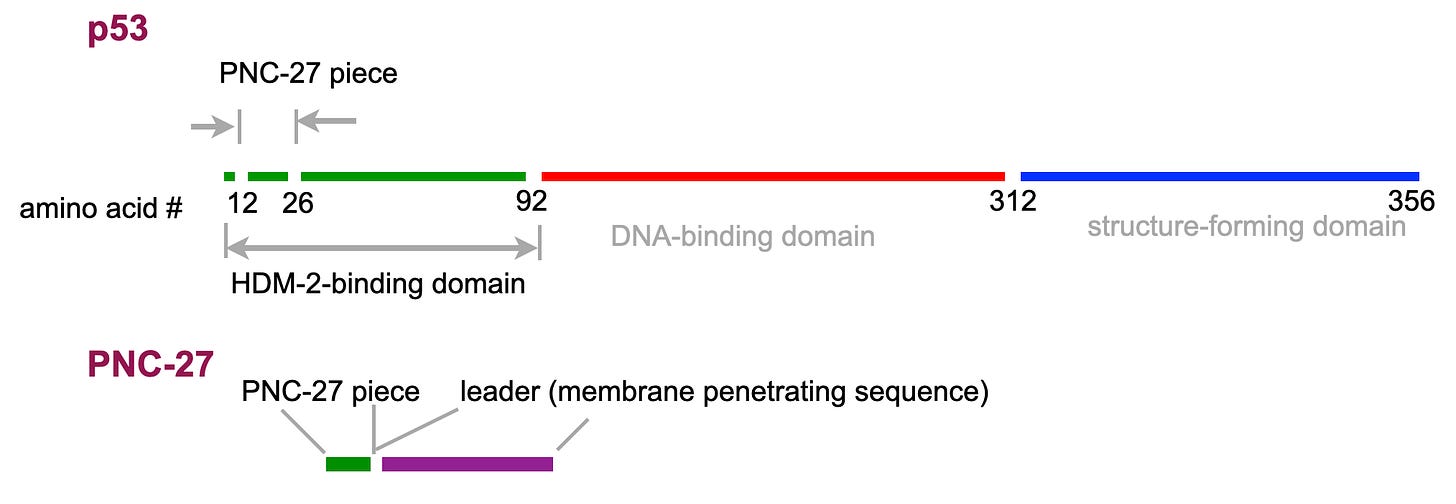
One of the most common tumor suppressor genes is p53. The gene has been found to be mutated in about 50% of human cancers. The gene product, the p53 protein, targets the cell cycle. The mutated protein causes the cell to get out of synch. This can be prevented if there is also wild-type p53 present. The normal p53 could thereby compete with mutant protein. Matthew’s idea was to make a small peptide that contained amino acids 12-26 found in p53. The peptide might compete with any mutant p53, allowing the native (wild-type) protein to get the normal cell system back on track. To make such a compound, in addition to synthesizing the peptide, it is also necessary to attach a signal that will allow the synthesized peptide to get into cancer cells. This peptide fragment is known as a “leader sequence” or “membrane penetrating sequence.” Matthew attached a leader sequence to the piece from p53. The fused peptide is called PNC-27.
When PNC-27 was tested, it turned out that it did not turn the tumor cells back to a normal state. Surprisingly, what happened was that adding PNC-27 to cancers cells in culture killed them dead. Many kinds of cancers — derived from breast, from colon, from liver. All kinds. Even cancers that did not express p53. This was clearly a breakthrough but not what was planned. Even more impressive, PNC-27 was without effect on normal cells. All kinds of normal cells. This is critical: It’s easy to kill cells. It’s what you can protect. In chemotherapy, it’s the controls that count.
Killing cancer cells.
So, an anti-cancer agent was discovered but it was not about p53. Or was it? PNC-27 was designed to look like p53. Pasteur’s statement that chance favors the prepared mind was in a public speech, so we don’t know the exact words. Most of the French versions say chance favors only the prepared mind — a stringent requirement if you want good luck. In any case, the normal p53 mechanism was known and this lead to the true mechanism in the synthetic peptide, PNC-27. The target of p53 protein is a nuclear protein called HDM-2 (note: this is the last abbreviation in the post). Leaving out some of the details, the mechanism involves the formation of a p53-HDM-2 complex making p53 protein susceptible to destruction and allowing cell cycle to proceed.. Matthew went after HDM-2. The search led to the remarkable discovery that HDM-2, a protein normally found only in the cell nucleus, appears, in cancer cells, on the cell membrane (the yellow blob in the figure below). Like p53 itself, PNC-27 went after HDM-2 — now on the outside of the cell. Surprisingly, the combination created a pore, a hole in the membrane, killing the cell. And normal cells, all kinds, didn’t show HDM-2 on their cell membrane or, at least, very little. That’s why normal cells are not killed by PNC-27.
Does PNC-27 kill tumors in vivo, in living organisms?
The answer is yes. For example: a highly metastatic rat pancreatic cancer was transplanted into “nude mice,” mice which do not have an immune systems that might otherwise reject the tumor cells. The mice developed tumors as expected. When treated with PNC-27 for two weeks, the tumors had disappeared. There were no tumors even after a prolonged period after treatment was stopped. Mice treated with control, an unrelated and inactive peptide harbored large tumors that metastasized.
In a similar experiment, human acute myelogenous leukemia (AML) cells were transplanted into the bone marrows of nude mice. (Again, no rejection of the AML cells). These mice developed AML and showed high white blood cell counts. Treatment for 3 weeks with PNC-27 resulted in normalization of their white blood cell counts. Bone marrow transplants were carried from the AML-PNC-27-treated mice into untreated nude mice (no AML, no PNC-27). The implanted mice had normal white blood cell counts and no evidence of AML. Both treated mice and secondarily transplanted mice showed dramatically increased survival times over mice treated with inactive control peptide. There were no signs of side effects.
Where we’re Going. PNC-27 and HDM-2. Therapy and Diagnosis.
The research plan in our lab: PNC-27 has proved to be effective in eradicating tumors in animal models and we know how it works and that it will not cause undesirable side effects — it doesn’t affect normal cells. We are actively seeking funding to enter human trials. The promise of the compound as a treatment for at least some cancers is great.
At the same time, as described above, the target of PNC-27 is the protein HDM-2. Unique to cancer is the appearance of the protein on the exterior cell membrane suggesting identifying HDM-2 on a cell may be diagnostic for the cancerous state. We and others have shown this in several different cell cultures and there is the likelihood that this can be adapted to human tests that are currently not definitive. Our first target is human cervical cancer which is diagnosed by taking scrapings of cervical tissue, placing them in preservative fluid, applying the solution to slides, staining the slides with a Papanicoleau (Pap) stain and then examining the slides microscopically. This is subject to error. Examining Pap smear samples for HDM-2 in the membranes of the cervical cells may allow us to diagnose cervical cancer directly, rapidly and more reliably.
Finally, the enhancing effect of ketone bodies suggests that many known anti-cancer drugs or other modalities can be used at lower doses thereby reducing side effects and toxicity.
Another major consequence of our studies is that we can lower the effective doses of chemotherapeutic agents by administering them with ketone bodies. We are developing methods to treat cancers such as cervical and skin cancers with externally applied solutions of PNC-27 in lithium acetoacetate to eradicate these tumors. Watch this space.







Oh this is a pretty fresh post, really insightful. I have known about PNC 27 for many years but never found a good description of how it works.
I've followed your work for many years. Glad to see this update, and glad to see you on substack.