Ultra-processed food, Darwin's “wrong” beans and oxidation-reduction reactions. Part I.
Scientific communication. What is the definition?
We expect some degree of precision in science. We expect unambiguous definitions. Colloquially, we must know what we are talking about. Science is a human activity and things go wrong. The phrase “ultra-processed food,” is everywhere, Substack posts, New York Times, scientific journals. The style, however — value judgements used as facts — suggests that if we search for a definition of “ultra-processed food,” we will not be satisfied. The original sense of the phrase implies that such foods represent risks. Insofar as there are experiments, the data suggest many are actually beneficial. The most common phrase in the discussion of the data is that there is “no exact definition.”
Nutrition has never been a sophisticated field and we tolerate poorly defined terms, or even biased definitions. (The studied diet may be “healthy” or “evidence-based.” By implication, alternatives are not). Surprisingly, words that are assumed to be understood but are poorly defined appear often in the history of science in more precise, that is, physical sciences.
Darwin: the oddest case which I have known.
The murky state of ultra-processed food brought me back to an episode described by Darwin in his autobiography:
“…the oddest case which I have known. A gentleman (who, as I afterwards heard, is a good local botanist) wrote to me from the Eastern counties that the seed or beans of the common field-bean had this year everywhere grown on the wrong side of the pod. I wrote back, asking for further information, as I did not understand what was meant; but I did not receive any answer for a very long time.”
Note: There are many odd things in biology but in case you are not familiar with botany: There is no such thing, in plant biology, as a “right” or “wrong” side of the pod. The comment is as weird as it sounds.
At some point, Darwin said:
“I saw in two newspapers, one published in Kent and the other in Yorkshire, paragraphs stating that it was a most remarkable fact that ‘the beans this year had all grown on the wrong side.’ So I thought there must be some foundation for so general a statement.”
“I went to my gardener, an old Kentish man, and asked him whether he had heard anything about it, and he answered, ‘Oh, no, sir, it must be a mistake, for the beans grow on the wrong side only on leap-year, and this is not leap-year.’ I then asked him how they grew in common years and how on leap-years, but soon found that he knew absolutely nothing of how they grew at any time, but he stuck to his belief.”
“After a time I heard from my first informant, who, with many apologies, said that he should not have written to me had he not heard the statement from several intelligent farmers; but that he had since spoken again to every one of them, and not one knew in the least what he had himself meant. So that here a belief–if indeed a statement with no definite idea attached to it can be called a belief–had spread over almost the whole of England without any vestige of evidence.”
This is played out again in ultra-processed food (UPF). There is extensive discussion in many different venues but there is little light — nobody knows what UPF is. Nobody can tell us that a substance can be a risk for something or other when no experiments have been done. How can you give something a name and then look for what that thing is? We can tolerate it — “we all know what it is.” But it is not benign. It becomes incorporated into official recommendations. Such undefined “beliefs” is that is hard to make them go away even after you’ve shown them to be unreal. And they may be genuinely confusing if you think there might actually be a meaningful unstated mechanism. Especially to students.
A modern example. A personal story.
It can be a problem in real science and I am one of those students who early on was confused. The issue: what is the definition of an oxidation-reduction reaction? What? Wasn’t that settled decades ago? To understand the confusion, let’s look at a truly informative evolution of definitions in the physical sciences.
Acids and Bases
Early in the history of civilization, people noticed that some substances were distinctly sour and gave them the name “acids.” Another class of molecules that came to be called “bases” felt soapy to the touch and had the property that they reacted with acids. The question was: what ias the defining characteristics? In other words, if I find a new compound, I want to know if it is in the class of acids and bases and thereby I can predict its behavior. The concept of acids and bases became refined but there were some odd turns. In the eighteenth century, Lavoisier noticed that many of the compounds called acids — sulfuric, carbonic, for example — contained oxygen and he thought that this was a general feature of acids. The connection is seen in the German word for oxygen, Sauerstoff, “acid stuff.” We know now that not all acids contain oxygen and the definition had to be expanded.
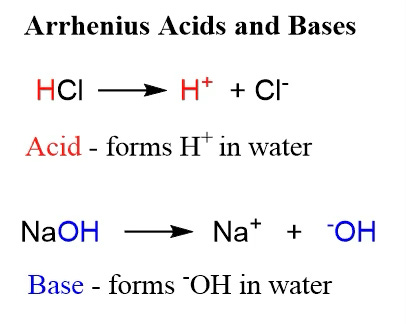
Once the concept of an ion was established, it became clear that acids were substances that were capable of ionization to produce H+. Hydrogen is the simplest atom and loss of its electron means that the hydrogen ion is just a bare proton, which is the way it is frequently referred to. The simplest bases were hydroxides and the key reaction, “neutralization,” was considered the reaction of a hydrogen ion with hydroxide ion. The model is attributed to the Swedish chemist Arrhenius.
Further refinement led to our modern definition of the so-called Brønsted-Lowry acids. We can expand the definition so that acids are proton donors in chemical reactions. Bases are proton acceptors.. This definition includes all the previous examples and some new ones.
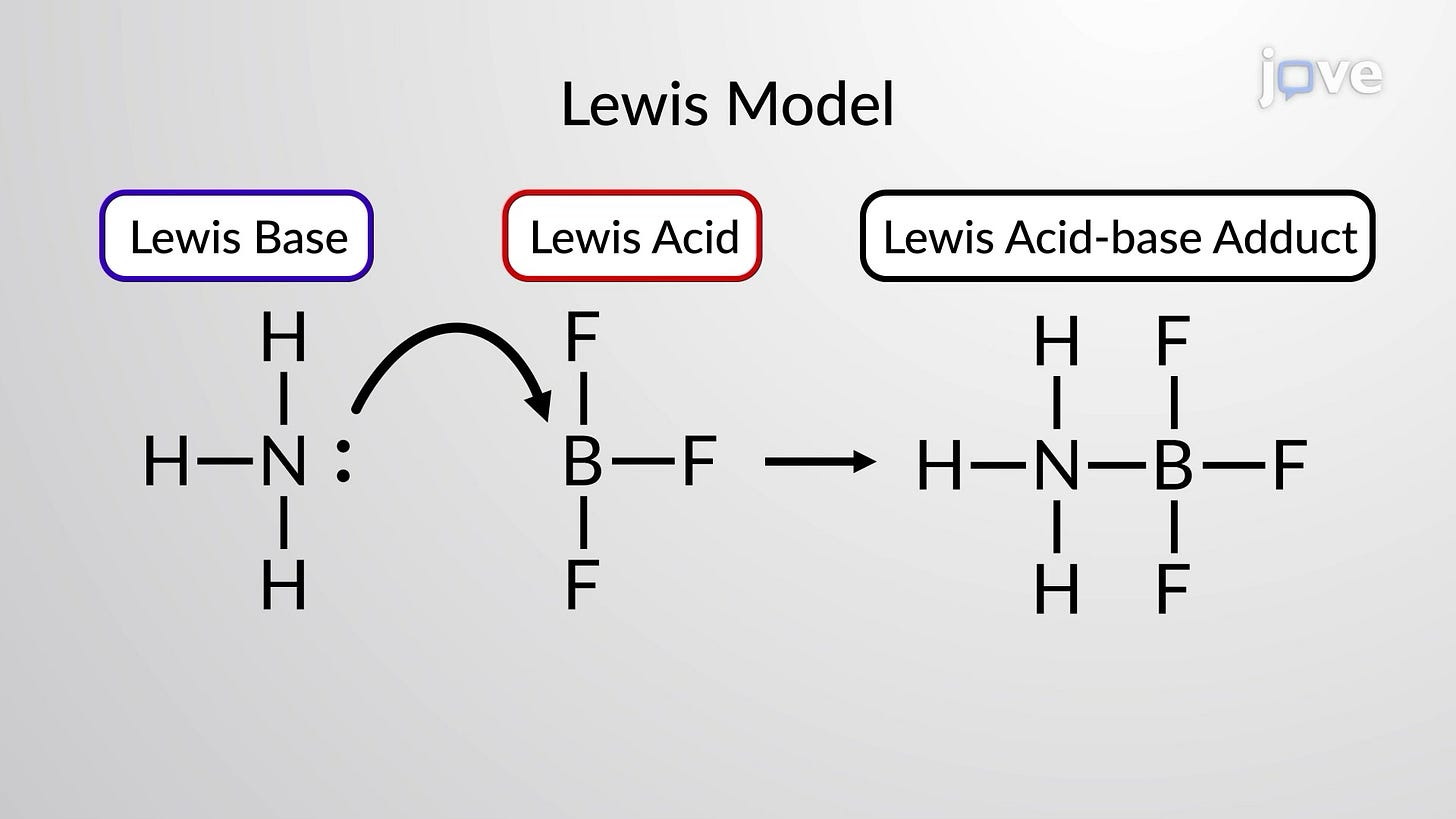
The idea became refined to a most sophisticated state: Lewis acids. The model saw the reactions in greater generality and the broader definition did not even require hydrogen. Lewis acids are substances that accept a pair of electrons. Lewis acids now include Brønsted acids and Brønsted bases.
The point was that progressive definitions included previous examples but refined the basis of the assignment. It impressed me as a child. It was the beauty of chemistry. I did not follow up much — I was not a child prodigy — but I loved the logic of it. When I studied oxidation-reduction reactions, I expected a re-make of the story.
Oxidation-reduction reactions
Now, originally oxidation was the name given to combustion or generally the combination with oxygen. The extension to combination with non-metals was clear but then something happened. I did not really understand the generalizations to oxidation numbers and the idea of partial transfer of electrons. Partial transfer of electrons? That’s the definition almost all chemical reactions — ionic reactions have complete transfer of electrons but that’s a small part of what we study. I considered it a limitation of my brain and went on with what I did understand. A half century later, I realized that there really was no thoroughly consistent definition. I sensed that this might be an insight, at least in education, and I presumed to bring it to the attention of the chemical education world. My colleague Gene Fine and I wrote a paper for the Journal of Chemical Education. It didn’t work out. Reviewers of the paper were actually somewhat hostile although, as in ultra-processed food and Darwin’s beans, they didn’t really have a definition. The story will be described in the next post.






Before I'd ever heard of "Ultra Processed Foods" I was warning patients to avoid "Ingestible Industrial Products" which masquerade as food.
There is unfortunately an awful lot of "everyone knows that" kinds of ideas when it comes to health.
Typo: "Saurestoff" should read "Sauerstoff".