A previous substack post described the history of PNC-27—an interplay of serendipity and a prepared mind—leading to the surprising result that a small peptide (15 amino acids) was able kill many different types of cancers in tissue culture. This is very surprising in that, as generally appreciated, cancer cells vary greatly, sometimes even within the same tumor. Beyond the promise of a magic bullet, knowing the mechanism might lead to the long sought after precise “hallmark of cancer,” as our previous generalizations were titled.
A series of papers by Matthew Pincus, Ehsan Yazdi and coworkers identified the mechanism. The PNC-27 peptide duplicated one of the binding domains of the p53 protein, the gene product of the most commonly mutated tumor suppressor gene. The idea was that PNC-27 would stand in for the mutated gene product and straighten out the defect. In fact, the compound did bind to HDM-2, a large intracellular protein, the known target of p53. However, unlike the anticipated effect on the complicated machinery controlling cellular metabolism, HDM-2 was found to be on the surface membrane of cancer cells, and PNC-27 never got into the cell. The PNC-27:HDM-2 complex formed a pore in the membrane, killing the cell. The figure below is a diagram of the PNC-27 peptide going after the now-membrane-bound HDM-2 molecule (the yellow blobs). Together, they make a hole in the cell membrane, leading to cell death.
Is this a general diagnostic method? Will PNC-27 kill cervical cancer cells?
PNC-27 was capable of killing many different cancers in culture—breast, colon, ovarian, and others—notably, cervical cancer. All of the cancer cells that were looked at were distinguished from normal cells by the appearance of HDM-2 on their external cell membrane, rather than being strictly inside the cell. Normal cells do not have HDM-2 on their external membranes (although all have the protein inside the cell), and they are not affected by PNC-27. This means that we have a potential process to identify cancer in a mixture of different cells; that is, we have a prospective diagnostic method. The paper we are submitting for publication describes the evidence for using the technique to screen for cervical cancer.
One of the common diagnostic methods for cervical cancer is the Pap test, or Pap smear. Because most cervical cancers are caused by human papillomavirus (HPV), an HPV test is sometimes used instead of or in addition to a Pap test. Cells are collected from the cervix and added to an alcoholic cytological preservative fluid medium, and the samples are then examined for cancerous or precancerous cells. Current methods for detecting cervical cancer have some problems. The Pap test, for example, may still reside in the visual morphology of the cells, and may depend on the judgement of the pathologist. In addition, well beyond my area of expertise, a vaccine against HPV has entered the fiery world of medical controversy. Our proposed diagnostic method would provide enhanced diagnostic accuracy, although the real world problems would still exist; for example, a major problem with Pap tests is that they are uncomfortable and sometimes painful, so people do not always have them done.
The proposed diagnostic method depends on this unexpected appearance of HDM-2 on the exterior cell membrane of the cervical cells. One way we can identify those cervical cells that are cancerous is to hit them with PNC-27 and show that the cells are destroyed. A method for showing that cells have been destroyed is to measure the enzyme lactate dehydrogenase, which leaks out of damaged cells. In the experiment, the cervical cancer cell line HTB35 was treated with PNC-27. PCS480, a normal cervical cell line that does not express HDM-2 on its surface, served as the control. The results, shown in the figure below, tell us that cervical cancer cells can, in fact, be killed by PNC-27. We also showed that the peptide is bound to HDM-2 on the cell membrane.
Using antibody methods, we were also able to show that PNC-27 does, in fact, directly form a complex with HDM-2. Antibody methods are cheaper and easier to do than using PNC-27, but they give the same results; both target HDM-2.
What would it take to have a diagnostic test?
The good news is that, after an analysis is carried out, the Pap test solutions are preserved, and some may be available. This means that we can determine whether the results we got in the lab will hold up when subjected to the conditions of a Pap test medium. In other words, can we use existing Pap tests for our assay? This method would not require any additional regulations on privacy and safety beyond the original Pap test rules. We have studies of this type. We used the technique of flow cytometry, which is pretty much what it sounds like: mixtures of cells flow through a tube containing a material that will allow different cells to pass through at different rates. We used a stain-labeled antibody method to identify the flow cytometry peaks.
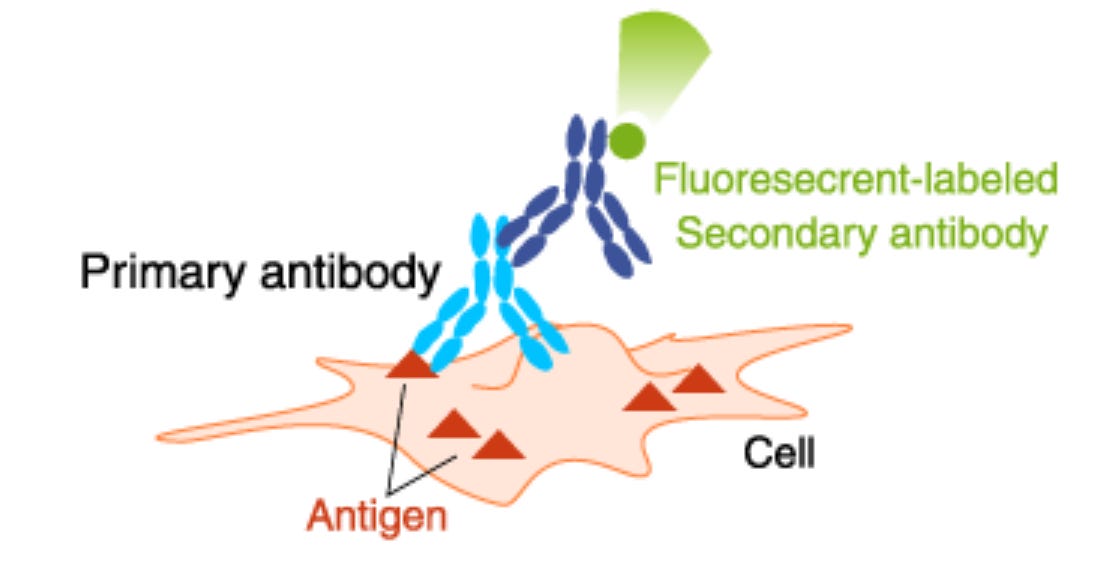
Brief description of an antibody technique for following cells.
A primary antibody recognizes a particular target (antigen) and is raised in a particular animal—say, a rabbit. In some cases, an indicator—a dye or enzyme whose activity can be measured—can be attached to this antibody. For several reasons, such as that the antibody may be difficult or expensive to produce or for amplifying the signal, a secondary antibody carrying the indicator can be added. We used a commercial rabbit anti-HDM2 antibody as the primary antibody. The secondary antibody was a commercial goat anti-rabbit antibody that was linked to a fluorescent dye.
HDM-2-bearing cervical cancer cells can be detected in Pap test solutions.
To test if the surface-bound HDM-2 could be detected in preservative fluid, cultured HTB-35 squamous cervical cancer cells and normal counterpart cells were placed in the cytologic preservative fluid, and flow cytometry was performed on the samples. The fluorescent antibody system was used to detect the cancer cells. Three different peaks were observed, indicating different cell samples separated by passage through the flow cytometer (the colors are solely for illustration). The cancer cells stained with the combined anti-HDM-2 antibody and secondary antibody with fluorescent indicator are shown in blue. The cells are clearly distinguished from those cells treated only with the secondary antibody with fluorescent indicator (green; not specific for HDM-2), as well as from cells that had not been treated at all (red). In distinction, in the right panel, normal cells show no effect of any treatment. The results show that flow cytometry can be used to detect squamous cancer cells in human material placed in liquid biopsy preservative fluid.
To be a fully functional diagnostic method, procedural details will have to be worked out, but we think this represents the essential feature of a new method for detecting cervical cancer.
In addition, looking ahead, many squamous cell cancers, including skin and cervical cancers, occur on external surfaces, making them susceptible to topical treatment. Since PNC-27 kills cervical cancer cells without affecting normal cells, topical treatment of surface squamous cell tumors could result in tumor eradication without off-target effects and systemic complications.






nice information. Hope you get the formal paper published.
A very detailed and well researched article! This is so valuable because 90% of sexually active people will contract HPV at least once in their lives. HPV touches the lives of almost everyone on the planet. So there can never be too many advocates for HPV vaccination, Pap smears or any other preventative strategies against HPV infection. Fantastic work!